This is a newspaper article predicting climate change in 1912. Not too shocking … from a chemical engineering perspective you can grasp the concept with a simple mass balance. The economic benefit from fossil fuels was so great that as a society we didn’t do anything about its very real negative consequences until recently. And now it’s the most important problem of our time. (Here is an explanation and Snopes write-up revealing this is, in fact, a real article.)
Author Archives: joshuagallaway
Happy New Year
I enjoyed the perspective(s) of this short Medium post
What’s next for batteries
In a new post on The Electrochemical Society’s redcat blog, Robert Kostecki, past chair of ECS’s Battery Division, talks about what’s next for batteries.
For Kostecki, the future of battery technology lies in reexamining past technologies with today’s tools. Advances in equipment and materials may mean that a technology ruled irrelevant in the past could be viable now with the aid of today’s more sophisticated technology.
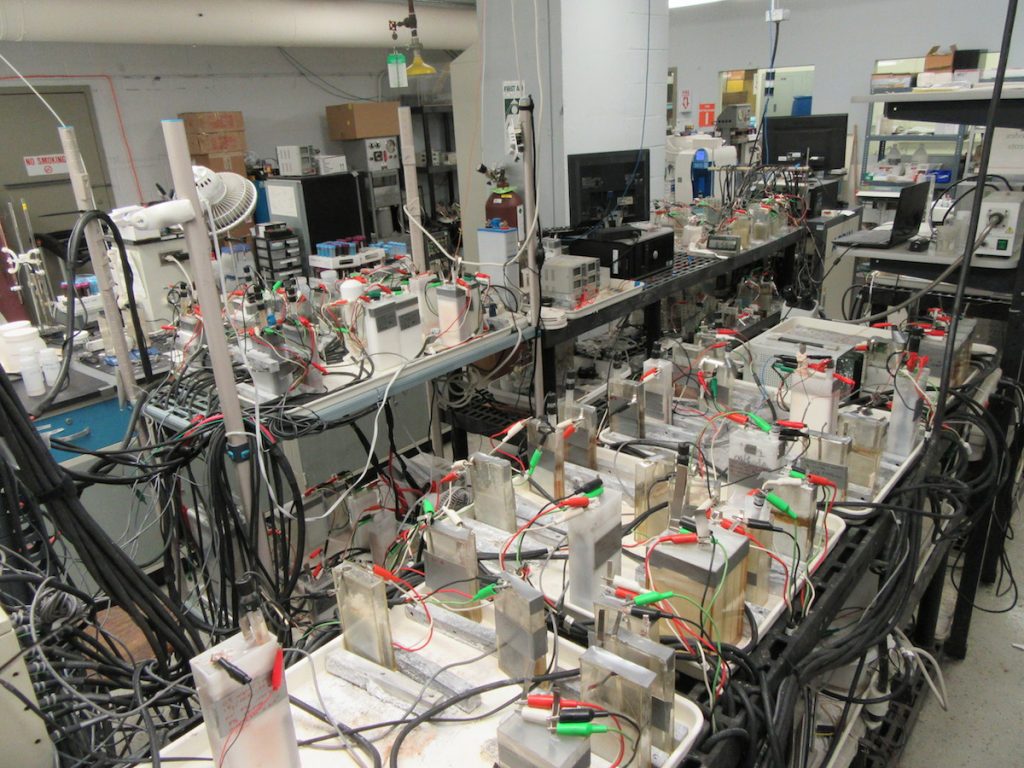
I couldn’t agree more. What he’s specifically talking about here is revisiting battery strategies that have been previously deemed not viable, such as multivalent ion, lithium metal anodes, lithium-air, etc. All those are undeniably important, but here at the CUNY Energy Institute we’ve also been re-assessing aqueous batteries. The reason for this is because they’re uniquely suited for an emerging market need: stationary grid-level batteries.
Specifically, we’ve used modern techniques to bring new understanding to the phase transformations thought to make MnO2 cathodes non-rechargeable. And in doing this, we’ve also figured out novel ways to cross those phase transformations reversibly. Some new papers are due out from us in the new year. Stay tuned.
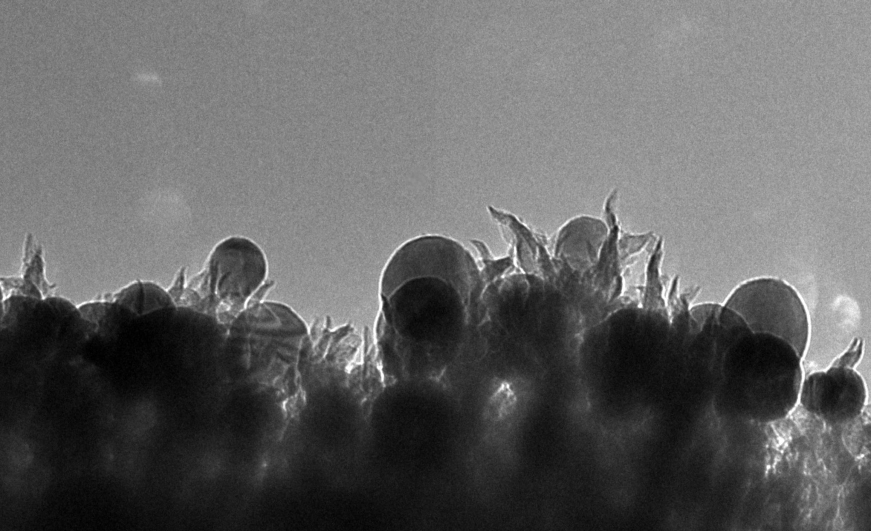
High-selectivity electrochemical conversion of CO2 to ethanol at Oak Ridge
A group at Oak Ridge has found a single catalyst that accomplishes the reduction of CO2 to ethanol at 65% yield, pictured above. The nanostructured catalyst is made of copper spheres embedded on carbon spikes. They argue that the activity is due to the electric field on the spike tips. The electrochemistry of ethanol is usually very complicated due to the carbon-carbon bond, so the fact that they form ethanol instead of methanol is quite interesting.
The paper is here in Chemistry Select.