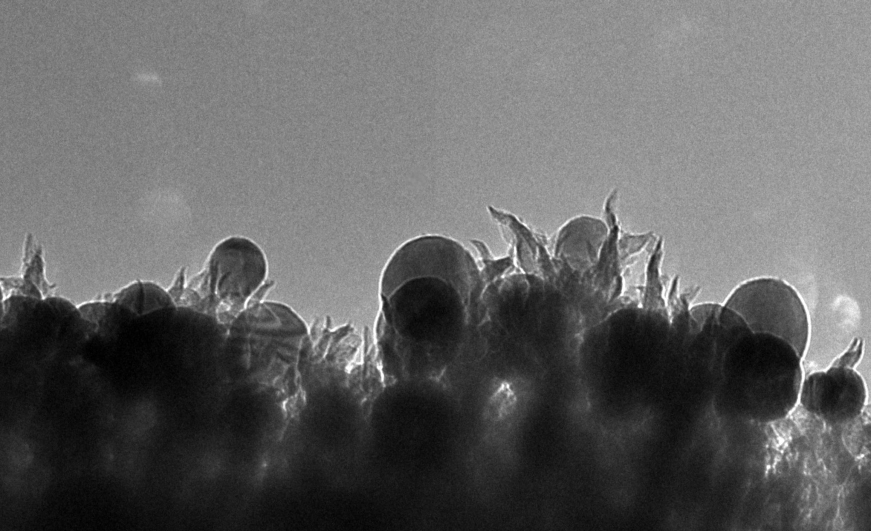
A group at Oak Ridge has found a single catalyst that accomplishes the reduction of CO2 to ethanol at 65% yield, pictured above. The nanostructured catalyst is made of copper spheres embedded on carbon spikes. They argue that the activity is due to the electric field on the spike tips. The electrochemistry of ethanol is usually very complicated due to the carbon-carbon bond, so the fact that they form ethanol instead of methanol is quite interesting.
The paper is here in Chemistry Select.