Author Archives: joshuagallaway
The beauty of dye-sensitized solar cells
Check out these photos of EPFL’s SwissTech Convention Center, which has a facade covered in dye-sensitized solar cells (DSSCs). The archetypal dye for a DSSC is a ruthenium complex called “black dye.” Dyes like actually have an enchanting purple-brown-black color, which isn’t totally black. But you can theoretically use any molecule that will inject an electron into a semiconductor when hit by light, and I just did a bit of hunting around and found a company called Dyenamo that specializes in different colors. The bottom picture is from C&EN, which has an article on non-silicon solar cells this week.
New PNNL paper on zinc/manganese oxide energy storage
I’ve had quite a few people email asking what I think about the new paper Reversible aqueous zinc/manganese oxide energy storage from conversion reactions in Nature Energy, by authors at PNNL. Understandable, since I spend a lot of my time talking about Zn and MnO2 for electrical storage. Fair!
First: unfortunately the work is being marketed (by PNNL) with a terrible graphic of a Platonic ideal supergreen™ battery that sits in a sunlit field and emits rays of light that save the world, but that’s pretty standard for battery research these days. Once the PR department gets ahold of it, you’re waist-deep in pictures of suns, windmills, iPhones, and Teslas. Most people, even most scientists, don’t understand the many levels of hierarchy involved in battery design and engineering, so I try to overlook these kinds of silly Photoshop excursions.
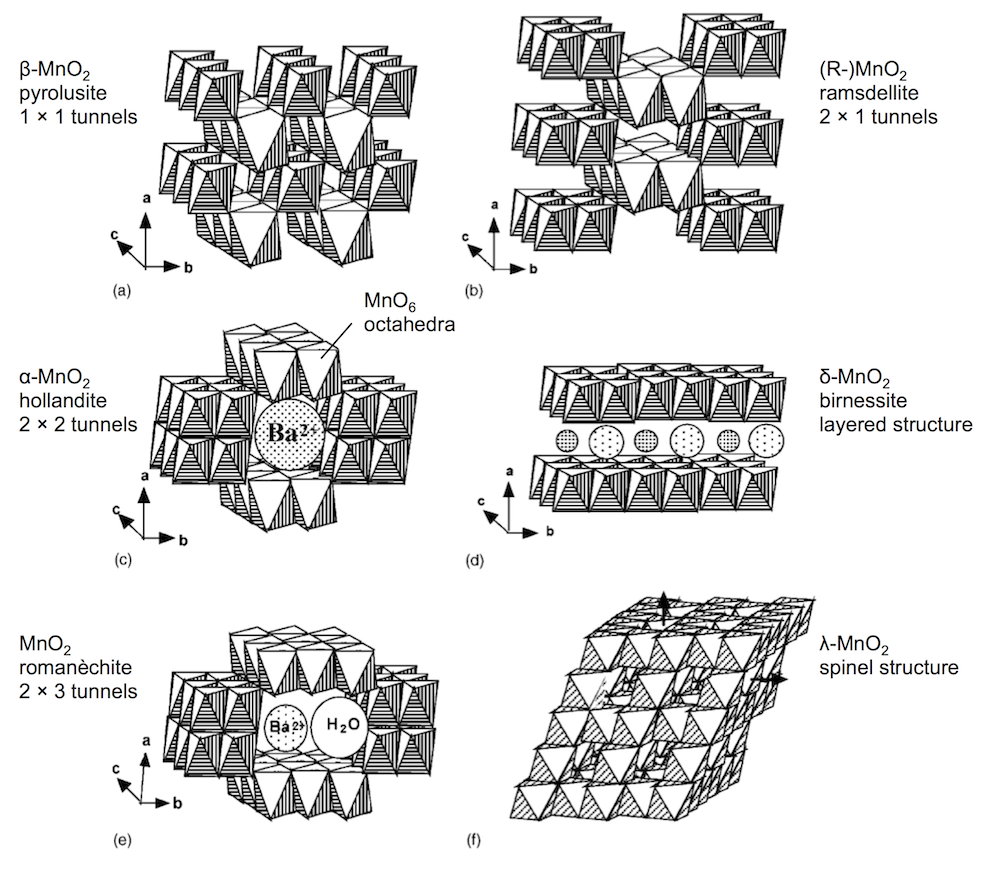
Second: the innovation of the paper is that they are making a rechargeable Zn-MnO2 battery in a mildly acidic electrolyte, and getting good cycle life. The way they’re doing this is by using α-MnO2 as their cathode active material. MnO2 comes in several polymorphic forms, some of which you can see below. (I adapted this figure from a paper by Poinsignon.) They are built from MnO6 octahedra, but can distinguished by the tunnel structures in the crystal.
A lot of my recent work has focused on the polymorph γ-MnO2, which is an intergrowth of (a) and (b) above. The PNNL work makes an interesting discovery about α-MnO2: they see the α-MnO2 going through a conversion reaction to MnOOH, which is somewhat unexpected. As you can see in the figure above, α-MnO2 is usually thought of as a host structure, to intercalate guest ions (like Ba2+). They then see that the surface of the MnOOH is coated with a large flake-like material that originates with the sulfate electrolyte, ZnSO4[Zn(OH)2]3 · xH2O. In this respect, the reaction is a bit reminiscent of a lead-acid battery, which also involves a sulfate film.
The paper is very interesting in that it provides unexpected evidence of α-MnO2 acting in the manner of a conversion reaction. (And that’s why that term is important and shows up in the paper’s title.) Also the zinc hydroxyl sulfate flake-film is a tantalizing look at what could be a very complex cathode reaction. And I’m a sucker for complex electrochemical reactions, as I hope you know. The test bed for the research was a CR2032 form factor, which is the kind of battery that goes in my running watch. So, the picture the PR machine and the science press are painting (with that world-saving battery up above) is a bit overblown, but the electrochemistry research underpinning this paper is extremely interesting, and I hope to see more.
Reactions at the single-molecule level
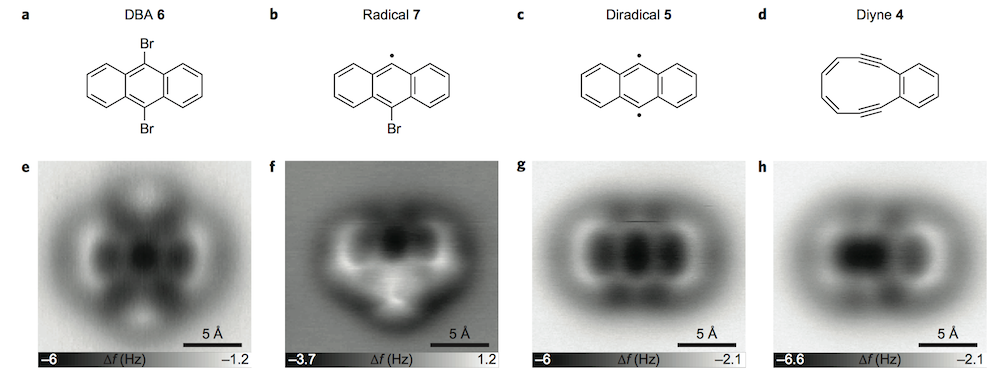
I’m always amazed by these studies where AFM and STM are used to watch cyclic molecules through a reaction. Here it’s used to image Bergman cyclization.
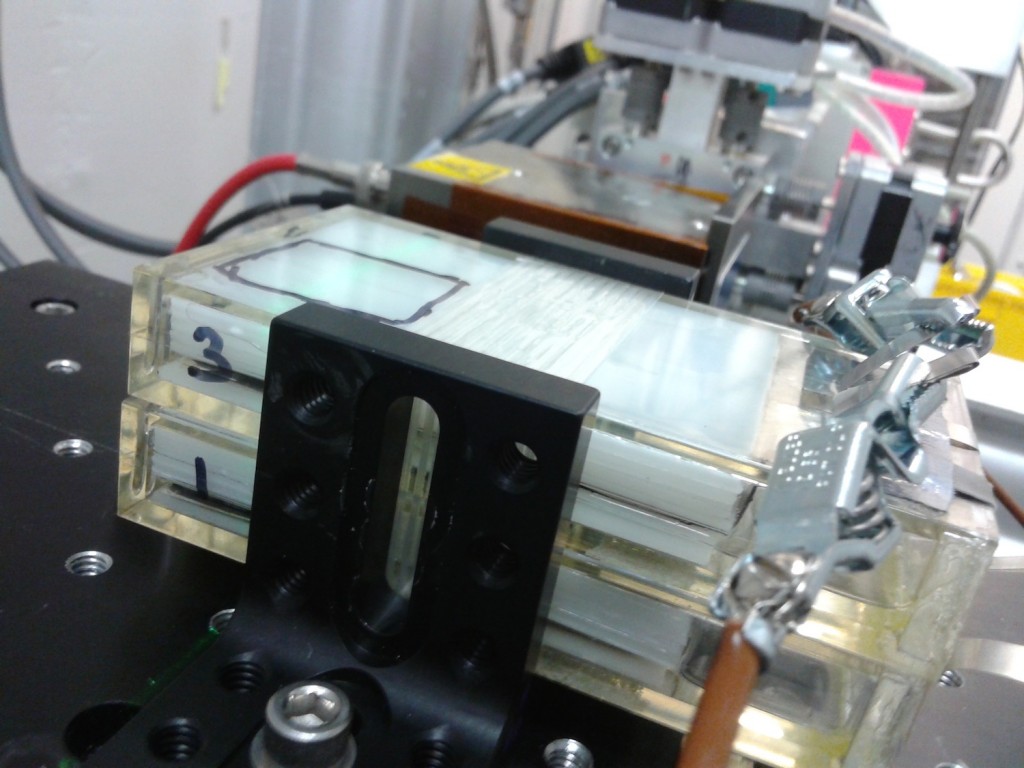
Battery testing at the Advanced Photon Source
Just returned from some battery testing at the Advanced Photon Source near Chicago. Here Snehal models one of the new batteries we’ve developed for grid-scale electrical storage.
And here’s a couple of them sitting in the path of an X-ray “white beam” that will give us diffraction data while they cycle. This way you can track the mechanism of what’s happening during battery cycling … since the cycling is ultimately an electrochemical transformation.
Now we’re going through a full set of data, getting to the bottom of our underlying mechanisms. By the way it snowed quite a bit while we were there. Here’s the lovely winter view from the guest house.