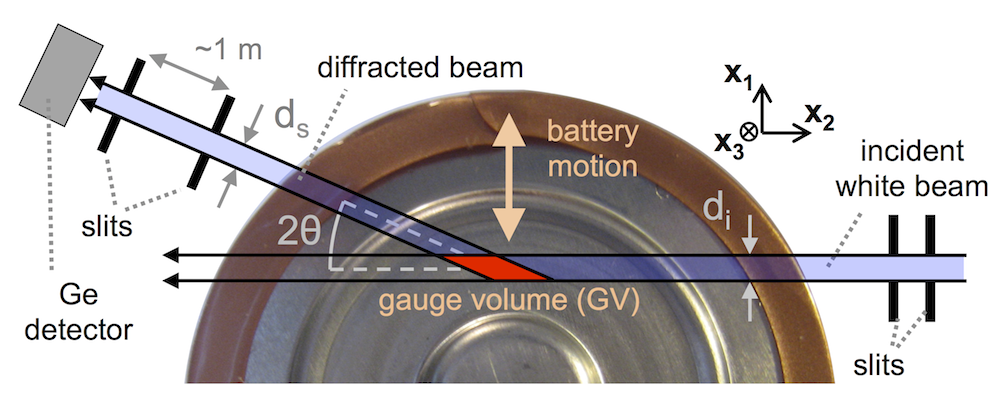
We have a new paper out, which describes work using high energy battery characterization to map the insides of batteries without opening them. “Hetaerolite Profiles in Alkaline Batteries Measured by High Energy EDXRD” is in the current issue of the Journal of the Electrochemical Society. The idea is to use X-rays with high energy and high flux to penetrate a thick battery and collect diffraction data. By setting slits to collimate the X-ray beam, a well-defined gauge volume (GV) is examined within the battery, as illustrated below:
Technically this is called energy dispersive X-ray diffraction or EDXRD. For each sample of data, you obtain three pieces of information: 1) Energy of the photons diffracted, 2) Intensity of the photons diffracted, and 3) Physical location inside the battery. This is plotted as a “diffraction contour” with energy on the x-axis, physical location on the y-axis, and log intensity of the diffraction as pixel darkness. As different crystalline materials diffract X-rays of different energy, you get an XRD fingerprint of the materials. Techniques like this are revolutionizing battery analysis, because physically opening a battery results in unacceptable mixing and oxidation.
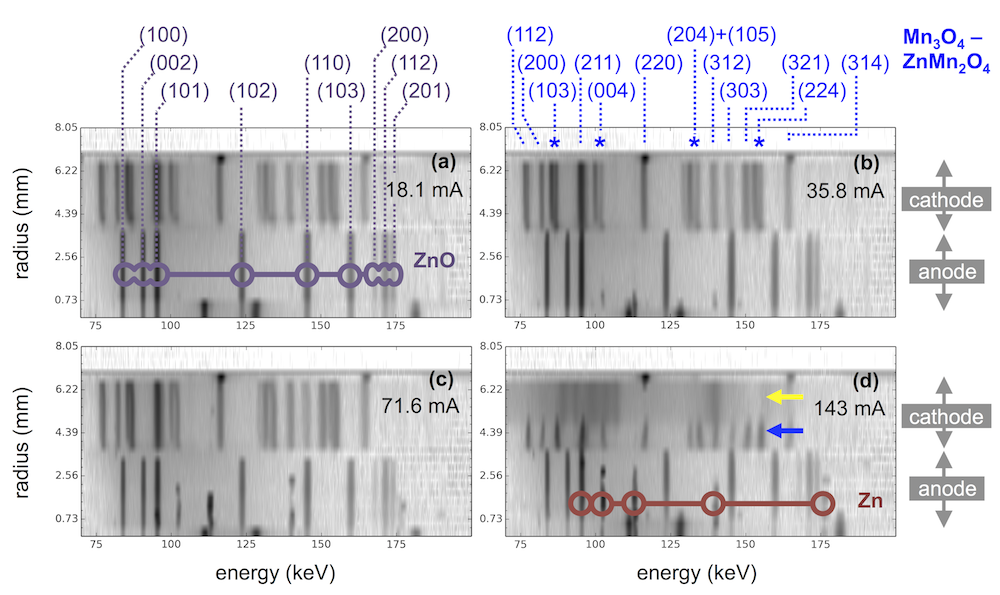
The plot above shows diffraction contours for four AA sized alkaline batteries discharged at different rates. The anodes are mixtures of zinc and zinc oxide, with higher current leaving more zinc behind. The cathodes are mostly mixtures of Mn3O4 and ZnMn2O4, which are nearly identical materials called hausmannite and hetaerolite. Even though these materials have structures differing by only 0.04 angstroms, EDXRD distinguishes them via peak splitting, marked above by blue stars.
The interesting thing is that the highest discharge rate (143 mA) has no hausmannite, but is instead split between hetaerolite and a different material, MnOOH. This shows that these two materials are the first to form, and that hausmannite is only a later product. As discussed in another recent paper, we want to know what reaction pathway leads to hetaerolite, because it limits the rechargeable performance of alkaline batteries. The results above provide the best clue we’re aware of, suggesting there is a groutite -> hetaerolite transition.