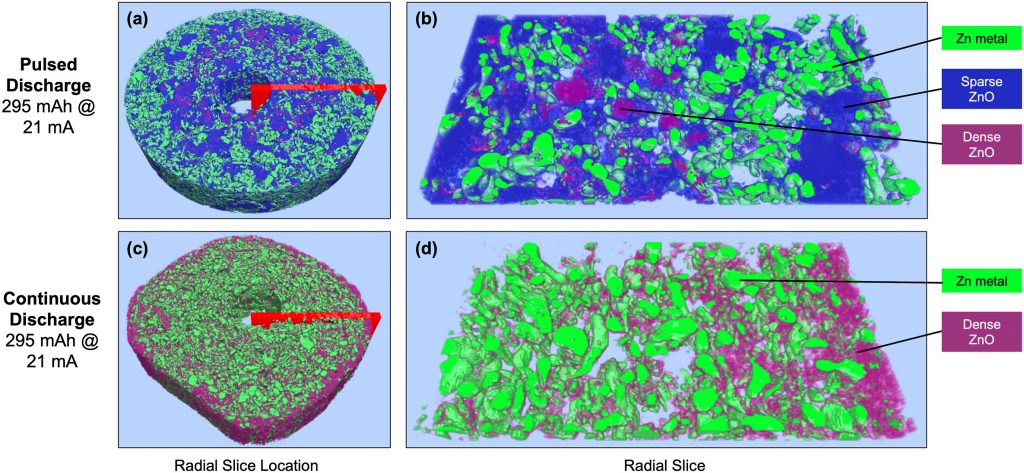
We have a new paper out in The Journal of Power Sources, which is a collaboration with Energizer. We use high energy white beam tomography to study the distribution of ZnO in the anodes of cylindrical alkaline batteries. (These are AAA, but we also studied AA sizes.) The finding is that the distribution of ZnO is a strong function of how the battery was discharged. In the image below, batteries (d) and (e) were discharged to the same depth (295 mAh) at the same rate (21 mA).
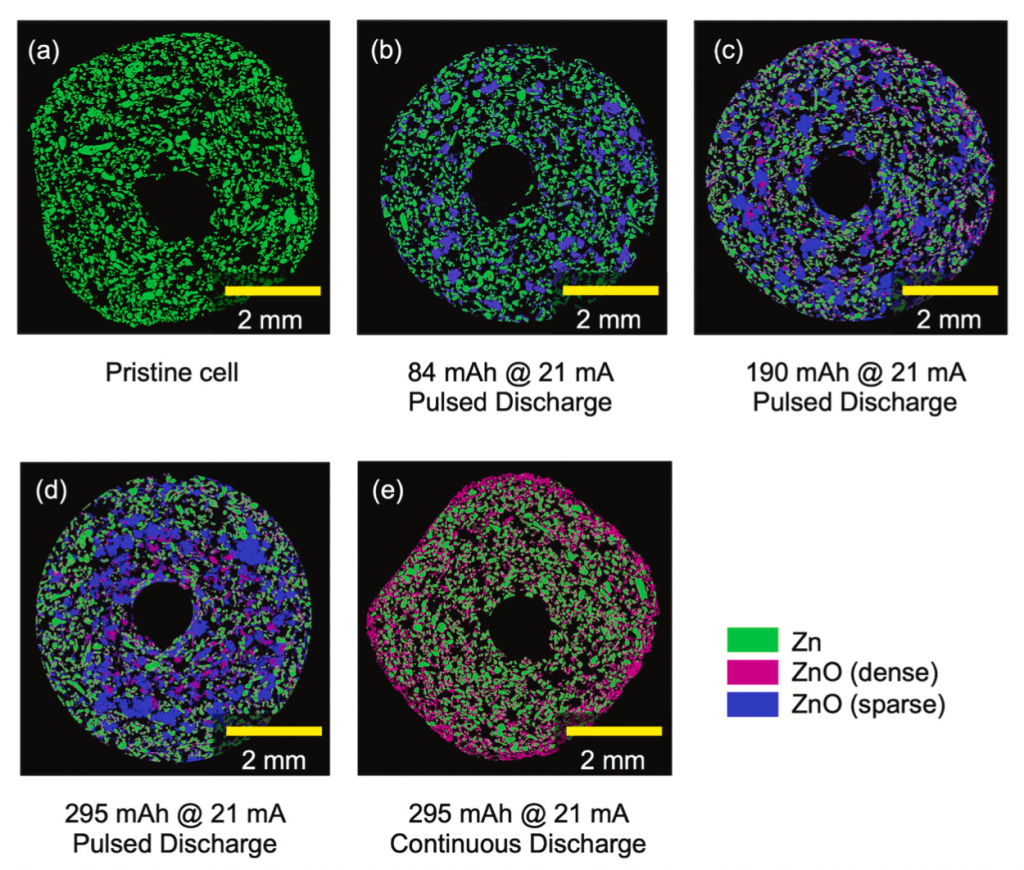
Continuous discharge (e) matched what you expect from a computational battery model: relatively dense ZnO (pink) found mostly near the separator, around the anode’s circumference. However, if the discharge was pulsed (d), the ZnO had a very low density (blue) and was mostly in large clumps in the anode interior. Since most primary batteries are used in an intermittent or pulsed manner, understanding how this affects where the resistive ZnO is located is important for getting more capacity out of the cells. We found that varying the ZnO density helps reconcile computational models with experimental results.
PhD student Dom Guida came up with a custom segmentation method to analyze these data. All the details are in the paper and the supplemental info. This tomography was special because the resolution was good (a little less than 3 microns) with a quite large field of view, letting us use unaltered AA and AAA cells and record the entire battery diameter.